Ecological
Theory and the Evolution
of
Complex Human Communities
William S. Abruzzi
Advances in Human Ecology 5:111-156
(1996)
ABSTRACT
Explaining social evolution has long been a central concern of anthropology. However, the discipline has yet to develop a systematic and testable model of social evolution that can effectively account for the differential development of complex human communities in diverse ethnographic contexts. This paper offers a model of community development based on the relationship between productivity, stability and diversity in ecological systems to account for the variable evolution of complex human communities. The utility of the model is then illustrated through its application to nineteenth century Mormon settlements in the Little Colorado River Basin.
Anthropologists have long been concerned with explaining social evolution (cf. Maine 1861; Tylor 1871; Morgan 1877; Spencer 1876; Frazer 1890; White 1959; Sahlins and Service 1960; Fried 1967; Flannery 1972; Adams 1976; Kottak 1982). However, despite more than a century of evolutionary thinking, anthropology as a discipline has yet to achieve a systematic and testable model of social evolution that effectively explains the evolution of complex human communities. Comparing the relative success and maturity of biological versus social evolutionary theory, Robert Nisbet (1969:227-28) noted that
"The differences between contemporary biological evolutionary theory and the biological theory of Darwin are immense. The difference between contemporary social evolutionary theory and the theory of Herbert Spencer do not seem very large or very significant."
Nisbet's observation still
applies, and the situation he describes has largely been given two general
explanations. The more common
claim is that human communities are inherently distinct from and more
complex than non-human communities and are, therefore, not as amenable to
strict scientific explanation. This
argument is a variant of the more general
Human
Exemptionist Paradigm (HEP),
which contends that human behavior is inherently different from that of
all other species and demands a qualitatively different form of
explanation (see Hardesty 1977; Catton and Dunlap 1980).
A central problem with the
HEP
is that the analytical distinction between human and non-human behavior is
proposed a
priori, rather than as the result
of a failure in applying comparable analytical methods to the study of
human and non-human communities. It
ultimately rests on what Leslie White (1949) called our
"anthropocentric illusion" of the uniqueness of the human
species. However, scientific
research has increasingly undermined the empirical basis of
anthropocentrism, and those individuals who claim that human behavior and
the evolution of human communities must be analyzed differently from that
of all other species are adopting a position that runs counter to the
scientific mainstream (see Cartmill 1994).
A second line of argument used
to explain the historical shortcoming of social evolutionary theories is
that they constitute at best poor analogies borrowed from the biological
sciences (Vayda and McCay 1975; Bennett 1976; Lees and Bates 1984; Smith
1984; Young and Broussard 1986). Eisely
(1958), Harris (1968), Stocking (1968), Nisbet (1969) and others have long
since exposed the fallacy of the thesis that social evolutionary theory
emerged as a stepchild of Darwinian evolution.
They have, in fact, demonstrated quite the opposite: that (1)
evolution is a concept with deep roots in Western thought, and (2) that
evolution eventually emerged as the prevailing paradigm of the biological
sciences only after it had thoroughly permeated most other fields of
inquiry, including the social sciences.
As Harris (1968; 122) points out, "Darwin's principles were an
application of social science concepts to biology."
Both Darwin and Alfred Wallace were strategically influenced by the
writings of Thomas Malthus (an economist), and it was Herbert Spencer (a
sociologist) who coined the term, "survival of the fittest",
which eventually became incorporated into the title of Darwin's chapter on
natural selection. Indeed, Harris (1968; 129) suggests that the term,
"Biological Spencerism... (represents) ...an appropriate label for
that period of the history of biological theory in which Darwin's ideas
gained their ascendancy."
The explanatory
limitations of social evolutionary theory do not, therefore, stem from the
inappropriate application of a biological metaphor, although the
superficial metaphorical use of biological concepts has all too frequently
occurred. Rather, the
deficiency results from the continuing failure of social evolutionary
theory to specify the significant characteristics of evolving societies
within an operational and theoretically coherent model of community
development that can be applied to a variety of local empirical
situations. The failure to
achieve this form of explanation ultimately derives from the application
of "Aristotelian" methods of explanation that have long since
been abandoned in the physical and biological sciences (cf. Lewin 1935;
Wilson 1969; see Abruzzi 2004). Like outdated Aristotelian explanations in physics and
biology, anthropological attempts to explain the evolution of complex
human communities have generally lacked the fundamental scientific concern
for applying a synthetic general theory to make testable predictions about
specific empirical developments within a local spatio-temporal context. For the most part, social evolutionary theory in anthropology
has largely consisted of empirical generalizations regarding the sequence
of qualitatively-defined developmental stages abstracted from the
ethnographic record (cf.Tylor 1871; Morgan 1877; White 1959; Sahlins and
Service 1960; Service 1971; Flannery 1972; Faris 1975; Rose 1981; Kottak
1982). However, empirical
generalizations do not constitute explanation in science (see Hempel 1965;
Nagel 1979). Rather, they
result only in "imperfect laws" (see Brodbeck 1962; Wilson 1969)
of social evolution, that is, those whose efficacy is based on statistical
correlations regarding the frequency of historical occurrences rather than
on their ability to provide a detailed consideration of a specific
empirical event. This
approach is clearly illustrated by Carneiro's (1962, 1967, 1968) use of
Guttman scaling to determine the "main sequence of cultural
evolution", as well as by White's (1959) "Law of Cultural
Evolution", Kaplan's (1960) "Law of Cultural Dominance",
Service's (1960) "Law of Evolutionary Potential" and many
subsequent attempts to propose laws of social evolution (cf. Flannery
1972; Faris 1975; Rose 1981; Kottak 1982).
However, the concern should be to develop "perfect laws"
that focus on the full concreteness of a specific situation.
When this this is the case, historical frequency no longer
determines the validity of a law. Lawfulness
exists not in the empirical association between historically connected
phenomena, but rather in the theoretical relationship between variables.
The historical occurrences themselves are not lawful; rather, they
are explained through the application of laws.
Anthropological explanations of social evolution have also been seriously handicapped by their widespread use of synchronic or cross-sectional data. This is a direct result of the typological orientation of social evolutionary theory and its traditional reliance on such questionable analytical procedures as the "ethnographic present" and the "comparative method". It is inappropriate to infer diachronic processes from the observation of synchronic data (see Barth 1967; Graves, Graves and Kobrin 1969; Plog 1973). Evolution is, by definition, a diachronic process and must be explained through the observation of time-structured information.
Anthropological
theories of social evolution have also been severely limited by their
reliance on cultures and societies
as basic analytical units (cf. White 1959; Sahlins and Service 1960;
Rappaport 1968; Bennett 1969, 1976; Flannery 1972; Leone 1979; Kottak
1982). Neither societies nor
cultures constitute viable analytical units for investigating social
evolution. To begin with,
societies and cultures are non‑operational concepts; they,
therefore, cannot be quantitatively linked to variations in specific
environmental or material conditions.
The selective forces that generate community development operate
upon individual populations adapting to specific local environments and to
the particular material conditions imposed upon them by encompassing
regional systems (Vayda and Rappaport 1968; Ricklefs 1987).
Although studies exist in which anthropologists have focused on the
developmental implications of local populations adapting to specific
material environments too often in such studies the environment has been
viewed qualitatively (as a "thing") rather than as complex and
dynamic multivariate system (Athens 1977; cf. Steward 1955, Sahlins 1958;
Netting 1968; Rappaport 1968; Bennett 1969; Meggers 1971; Leone 1979;
Kottak 1982). Consequently,
general models have not emerged from such studies: (1)
that systematically interrelate quantifiable environmental and social
variables within a predictive and testable theoretical framework; and (2)
that can be readily exported to a variety of distinct ethnographic
situations. Such models can
only be achieved when studies of social evolution concentrate on specific
local populations adapting to precise measurable conditions in their
material environments.
In the following
paper, I suggest that general ecological theory provides a useful model of
community development which, because it lacks the limitations inherent in
most traditional anthropological theories of social evolution, can be
applied to explain the evolution of complex human communities.
The proposed model is an adaptation of the general model developed
by plant and animal ecologists to explain the evolution of complex
multi-species communities. Before
I discuss the proposed model, it will be useful if I first address some of
the general issues surrounding the application of ecological concepts in
human ecology.
Ecology
and Community Development
Considerable
controversy surrounds the application of ecological concepts in
anthropological human ecology. Although numerous anthropologists have utilized ecological
concepts and principles to explain human social behavior (cf. Barth 1956;
Rappaport 1968; Gall and Saxe 1977; Leone 1979; Winterhalder and Smith
1981; Abruzzi 1982, 1987, 1993), others have rejected the strict
application of ecological concepts and principles to human populations
as naive and inappropriate uses of biological concepts (cf. Young 1974;
Richerson 1977; Vayda and McCay 1975; Bennett 1976; Lees and Bates 1984;
Smith 1984; Young and Broussard 1986).
Disagreement over the application of ecological concepts and
principles to human populations has even divided anthropologists who adopt
an explicit ecological orientation (see Moran 1984).
Those ecological anthropologists who view themselves as human
ecologists generally see ecology as providing a testable framework for
analyzing both human and non-human social behavior within a single unified
theoretical perspective. By
contrast, those ecological anthropologists who view themselves as cultural ecologists are more likely to reject the strict application
of ecological concepts and principles to human communities on the grounds
that culture acts as a mediating force which renders human adaptation
analytically distinct from that of all other species. For cultural ecologists, ecology serves as an orientation for
the study of human-environmental relations rather than as a set of
operational principles that can be used to explain specific human social
behaviors.
Ecological
concepts have, indeed, been misused in anthropology.
However, their misuse has occurred not because such concepts are
inherently inapplicable to human communities, but rather because they have
largely been applied incorrectly. For
the most part, ecological concepts have been extended to human communities
wholly disconnected from the encompassing theoretical systems from which
they derive both their meaning and their utility.
This is nowhere more clearly illustrated than in the historical use
of such concepts as niche
and ecosystem
in the social sciences. These two concepts have generally not been viewed
in dynamic and multidimentional terms, but rather have been applied mostly
as metaphors within a largely functionalist view of human‑environmental
relations (cf. Barth 1956; Rappaport 1968; Leone 1979; see Vayda and McCay
1975; Smith 1984; Catton 1993, 1994).
In addition, the term "ecology" has mostly been used in
the restricted substantive
sense in social analysis to refer simply to the relationship that exists
between a human population and its natural environment.
It has not primarily been applied formally
as a body of general theory leading to testable predictions regarding the
organization and evolution of specific local human communities.
The purpose of this paper is to supersede a metaphorical and
environmentalist approach to human ecology by demonstrating that general
ecology provides a meaningful and productive theoretical framework for
explaining the evolution of complex human communities.
My application
of ecological theory to human communities rests on several interrelated
assumptions (see Abruzzi 1982:13-14, 1993:12-14):
(1) that human communities are ecological communities through which
energy flows and by which population/resource relationships are regulated1
(see Margalef 1968; E. Odum 1971; H. Odum 1971; Little and Morren 1976);
(2) that any system containing living organisms constitutes an
ecological system (see Margalef 1968; H. Odum 1971); (3) that both human
and nonhuman communities contain a high degree of functional diversity
which is ultimately dependent on continuous inputs of energy from external
sources (H. Odum 1971); (4) that both human annd nonhuman communities
contain organizational units which vary in size and composition as a
result of spatiotemporal changes in the abundance and distribution of
resources (see Wilson 1968; Kummer 1971; Abruzzi 1979, 1982) and (5) that
those processes which underlie the division of labor (i.e., resource
partitioning) are central to the evolution of both types of communities
(cf. Harris 1964; Blau 1967; Levins 1968).
Furthermore,
while the properties of any particular ecological community are determined
by its specific biological composition, the laws or principles which
determine community evolution are inherent in the energetic (not
biological) relationships which exist within and between systems subject
to natural selection. Consequently,
the principles which determine the organization and evolution of
ecological communities apply to all
ecological communities
regardless of their specific biological composition, including terrestrial
and aquatic communities, single and multi-species communities, and
human
and non-human communities.
An industrial city is, therefore, just as much an ecological system
as is a tropical rain forest, a coral reef or a temperate grassland
community. Regulated by
energy flows that determine population distribution and functional
specialization, the settlement pattern and community organization that
evolve in industrial-urban communities are distinct from those found in
human communities based on irrigated agriculture, nomadic pastoralism or
hunting and gathering.
It is also
scientifically preferable to approach human social systems as a subset
of more general ecological systems, subject to the same theoretical
principles, than to continue to regard human communities as analytically
distinct from all other social systems. From the perspective of theory development, it matters less
whether human and nonhuman communities are substantively distinct, than
whether general ecological concepts and principles account for comparable
empirical developments in both types of systems.
Just as Newton's development of the
inverse
square law
eliminated the arbitrary Aristotelian distinction between celestial and
terrestrial motion (see Greider 1973:71-77) and the advent of Darwinian
evolution removed the equally artificial distinction between human and
nonhuman species in explaining biological evolution, so also does a single
explanation for the organization and evolution of human and nonhuman
communities provide a more parsimonious and powerful explanation for the
evolution of complex ecological systems than the perpetuation of two
distinct explanations: one for human communities, and one for the
remainder of the organic world.
However, if the
application of general ecological concepts and principles to human
populations is to prove useful, it must go beyond the simple relabeling of
social phenomena with ecological terms or the mere use of ecological
metaphors. It must be based
on a recognition that similar processes
operate in physically distinct and unrelated systems (see Ashby 1956;
von Bertalanffy 1968; Day and Grove 1975; Rapport and Turner 1977;
Alexander and Borgia 1978; Abruzzi 1982).
Furthermore, the central goal must be to determine whether the
specific concepts and principles used to explain the evolution of complex
nonhuman communities can be applied to account for specific empirical
developments associated with the evolution of human communities as well.
Such an approach is systemic
not reductionist. However, to
achieve this goal, ecological concepts and principles must be applied
within a predictive and testable theoretical framework.
It is only when the processual models of general ecology are
applied formally and explicitly to human communities that testable
predictions can be generated and that the applicability of these models
can be objectively evaluated. Consequently,
in order to explain (and not merely describe or heuristically
illustrate) patterns of community development, ecological theory must
account for specific empirical developments as a consequence of
predictions derived from general theoretical considerations, recognizing
that all general theoretical concepts and principles must be modified
and operationalized to fit a specific empirical problem or context.
In the next section of this paper, I outline the general features of the ecological model in question. Having completed this, I will then conclude with a brief discussion of the ways in which I have used this model to account for specific historical developments associated with Mormon colonization of the Little Colorado River Basin.
THE
EVOLUTION OF COMPLEX ECOLOGICAL COMMUNITIES
An ecological
community is defined as a set of interacting populations that exists
within a prescribed territory. The
evolution of complex ecological communities is the organizational process
whereby a growing population adapts to changing conditions of resource
availability created in part by its own growth (see Brookhaven National
Laboratory 1969; Whittaker 1975; Cody and Diamond 1975). Based on the principles of energy maximization that apply to
all living systems subject to natural selection, the ecological theory of
community development provides a set of general principles from which all
community organizational characteristics can potentially be explained.
The specific model employed here concerns the relationship between
population growth, community productivity and functional community
diversity and systematically connects changes in these three community
parameters with variations in resource availability
(see
Figure 1).
Figure 1
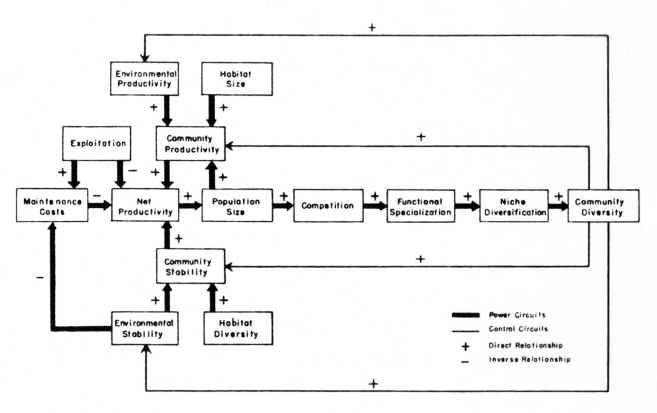
The Ecological Model
Understanding
the niche concept is central to
explaining the evolution of complex ecological communities.
The niche encompasses
several dimensions of a population's existence that affect its
contribution to the total flow of resources through a community (see
Levins 1968; Vandermeer 1972), including:
(1)
its habitat (spatial location),
(2)
its functional role within the community (including both consumptive and
nonconsumptive behaviors), and
(3)
its distribution along environmental gradients. From an energetics perspective, the niche is a function
performed within an ecological community that facilitates the flow of
resources among that community's constituent organisms.
A population's niche may be divided into its fundamental niche
and its
realized
niche (see
Vandermeer 1972:110-111). The
former comprises the exploitative position occupied by a population
within a given territory in the absence of competition, whereas the latter
consists of that portion of the fundamental niche actually filled by a
population in a particular community containing a specific set of
competing populations.
Competition is
the principal agent determining niche breadth in ecological communities.
Where two populations are complete competitors and one is dominant over
the entire niche, the less efficient competitor will be completely
eliminated from the arena of competition (see Gause 1934; Hardin 1960).
Where two populations vary in their relative competitiveness in
different portions of the niche, on the other hand, complete exclusion may
not occur. Each population
may evolve instead to occupy a more restricted realized niche when the two
populations occur within the same community (cf. Crombie 1947; Brown and
Wilson 1956). As additional
populations enter the competition, species specialization and niche
differentiation increase, and each population eventually comes to occupy
an increasingly reduced portion of its fundamental niche (Vandermeer
1972). Such resource partitioning among competing populations is central
to the evolution of complex ecological communities.
An explanation of community evolution must, therefore, focus on the
various conditions that either facilitate or inhibit the developmental
process.
Subsidies
and
Drains in Community Evolution
Resource
partitioning and niche differentiation in ecological communities results
directly from the competitive advantage accompanying resource
specialization. Species
populations which exploit a limited set of resources tend to be more
efficient in obtaining those resources than populations which must exploit
a broad range of resources for their survival (see Levins 1968:10-38;
Vandermeer 1972:114116). Consequently,
as additional species enter a community, niches are "squeezed",
and the range of resources exploited by individual populations within the
community is reduced. The
evolution of complex multispecies communities is, thus, both an incessant
and an opportunistic process through which natural selection generates the
greatest functional diversity possible within the limits imposed by
resource availability.
Ecological
communities depend upon the existence of abundant supplies of potential
energy in their environments in order to survive, and their complexity
increases to the extent that this potential energy can be converted into
community productivity, biomass (population)
and, ultimately, functional diversity.
However, only a small fraction of available potential energy can be
utilized by a community. The
critical factor determining how much potential energy is converted to
productivity is the total cost of maintaining organisms within a
community. A cumulative
reduction occurs in net productivity as energy flows from one trophic
level to the next, due to the cost of maintaining organisms at each
trophic level. This results
in the trophic pyramid that characterizes all ecological communities.
Maintenance
costs are, thus, the principal factor limiting the amount of energy
transferred between trophic levels. They,
therefore, directly affect both the amount of biomass and the level of
species diversity that can be supported within a multi-species community.
A decrease in maintenance
costs: (1)
increases net productivity at each trophic level;
(2)
increases biomass and niche differentiation; and
(3)
increases the viability of more marginal niches. Consequently, it
increases community diversity. An
increase in maintenance costs, on the other hand, decreases the
total amount of energy flowing through the system. It, therefore,
decreases supportable biomass, decreases niche differentiation, decreases
the viability of marginal niches, increases the likelihood of local
extinction and, therefore, decreases community diversity.
Any energy
source that reduces maintenance costs within a community increases the
total amount of energy that can be converted to community productivity.
Such a source serves as an
energy
subsidy to the development of that community.
Conversely, any energy source that increases community
maintenance costs diverts energy away from a community and imposes a
stress or energy drain upon that
community. Energy drains
reduce the total amount of energy converted to community productivity and,
thus, available to support niche diversification (see E. Odum 1971:43‑53
for a discussion of energy subsidies and drains in ecological systems).
Since the evolution of complex ecological communities ultimately
depends on the availability of resources, all phenomena affecting
productivity and energy flow in ecological systems may be viewed within an
energy subsidy/energy drain perspective. However, research has shown that certain environmental
conditions influence the evolution of complex ecological communities more
significantly than others. These include:
environmental
productivity,
environmental
stability,
habitat
size,
habitat diversity,
and
exploitation.
Environmental Productivity.
An
increase in environmental productivity raises the probability that a
sufficient abundance of resources will exist within an ecological
community to support a particular species population or adaptive
specialization. Conversely,
because a reduction in environmental productivity decreases the adaptive
or competitive advantage of specialization, it reduces community
diversity. For this reason, organisms and populations in less productive
environments must exploit a wider range of resources than those in more
productive ones. The generally high species diversity found in tropical
communities and in communities at lower versus higher elevations derive in
large part from the typically higher productivity associated with their
encompassing ecosystems (cf. Rosenzweig 1968, 1976; Terborgh 1971).
The direct
association between environmental productivity and community diversity may
be compromised, however, by the existence of specific limiting factors,
such as the rate of energy conversion associated with organic pollution (Odum
and Pinkerton 1955) or the deficiency of oxygen that characterizes many
highly productive eutrophic lakes (see Sanders 1968:267).
In addition, purely random perturbations in resource availability
may negatively affect species diversity in highly productive communities
by increasing the probability that more marginal niches will be eliminated
(MacArthur 1972:95; Rosenzweig 1976:129-130).
Environmental Stability.
Notwithstanding the
significance of environmental productivity, environmental stability is
perhaps the single most important factor influencing community diversity.
Unstable environmental conditions can frequently offset the
positive effect that high environmental productivity has on community
development (cf. Sanders 1968; Slobodkin and Sanders 1969; MacArthur 1972;
May 1973; Leigh 1975). Where
environmental instability prevails to the extent that substantial
resources must be expended just to maintain or replace existing organisms
within a community, little energy remains to support increasing
specialization and niche diversification.
While resource abundance and reliability permit an increase in
biomass, species specialization and niche differentiation, fluctuations in
resource availability reduce the viability of marginal adaptations and
reverse the effect that competition has on niche differentiation.
Species specialization, thus, serves as a reliable indicator of
community stability (Leigh 1975:56).
The same conditions determine the diversity of social organization
in single-species communities. Rapport
and Turner (1977:330) report, for example, that among social insects
"a fluctuating environment can make a particular caste uneconomical
and favor generalists over specialists even if the functions the caste
performs remain as important as before" (see Wilson 1968, 1971).
Environmental
fluctuations may vary in
amplitude,
frequency and
predictability.
The extreme temperature oscillations that occur in arctic
ecosystems produce high maintenance costs and result in the low species
diversity characteristic of polar communities.
Similarly, an increase in the frequency of fluctuations reduces the
time available for the evolution of complex energy-flow networks.
In his comparative examination of species diversity in benthic
communities, Sanders (1968) determined that increased diversity was
consistently associated with reduced seasonality. The most significant aspect of environmental variation,
however, is its predictability. Slobodkin
and Sanders (1969:85‑86) maintain that, even where an environment
oscillates, if it
Since a high
degree of specialization can only evolve within a highly predictable
environment, the most diverse multispecies communities occur in highly
predictable environments with low variability.
Thus, the greater diversity of tropical ecosystems derives more
from environmental stability than from abundant productivity.
Indeed, Sanders (1968) determined that species diversity was not
only greater in more stable benthic communities within the same climatic
zone, but also that it was greater in communities situated in stable
temperate ecosystems than in communities located in unstable tropical
ones. Notably, the most
complex community observed by Sanders was the shallow water community in
the Bay of Bengal, which is a productive and
stable tropical benthic ecosystem.
Habitat Diversity.
Habitat
diversity is also an important
factor influencing community evolution.
Environments differ in the degree to which resources are evenly
distributed and may vary from having resources that are uniformly spaced
(i.e.,
fine-grained)
to those that are patchily distributed (i.e.,
coarse-grained)
(see Levins 1968:10-38; Vandermeer 1972:114-116). Habitat diversity (i.e., a coarse-grained distribution of
resources) increases the likelihood of niche differentiation and enhanced
species diversity due to the greater efficiency of specialized resource
exploitation in coarse-grained environments.
Several studies have linked species diversity to environmental
heterogeneity, including Pianka's (1967) study of lizard species diversity
in North America and MacArthur and MacArthur's (1961) analysis of the
diversity of bird species in tropical habitats.
Habitat
Size.
To
the extent that environmental diversity is related to the size of the
physical area encompassed by an ecological community, an increase in
habitat size is also related to community diversity. The adaptive advantage of resource specialization in
coarse-grained environments only exist to the extent that the resources
provided by differentiated habitats are sufficient to support particular
populations and adaptive specializations.
Such conditions are simply more likely to exist in larger habitats.
Exploitation.
Exploitation occurs whenever one ecological system serves as an energy
subsidy for the maintenance or growth of another system.
Exploitation imposes an energy drain on the system being exploited,
because the productivity upon which community evolution depends is removed
from the exploited community. Human
populations pose a significant source of exploitation in multispecies
communities. However,
wherever one draws boundaries in nature, an unequal exchange of energy
flows across that boundary which contributes to the organizational
difference between the respective systems (see Margalef 1968).
A predator exploits its prey, and a herbivore exploits green
plants. In both situations,
the energy exchanged between organisms is unequal, and one system benefits
at the other's expense. The
same exploitation occurs between ecological communities, and the evolution
of complex communities can only proceed after their exploitation has been
discontinued.
Regulation
in Ecological Communities
Because stability
increases the efficiency of resource exploitation, natural selection
favors those mechanisms that reduce resource fluctuations within a
community. A selective
advantage, thus, exists for enhancing the control of regulating mechanisms
which render ecological communities increasingly independent of
immediate, short‑term fluctuations in their environment.
Regulating mechanisms in ecological communities may be divided into
power
circuits and control
circuits (H. Odum 1971:94).
Power circuits are the major channels of energy flow which
primarily determine a community's organizational structure as, for
example, where oak trees process most of a forest community's energy
budget. Control circuits
yield only minor energy flows, but are capable of affecting the flow of
energy in the substantially larger power circuits.
This occurs, for example, when the gathering and planting
activities of squirrels influence the size of an oak population.
Control circuits are
particularly important for the work-gate functions they perform (see H.
Odum 1971:38, 44-45), wherein one energy flow is enhanced by the
multiplicative effect of a supplementary energy input. Agricultural
practices such as weeding, plowing and irrigation perform work-gate
functions in that they augment the flow of energy that becomes stored in
consumable plant material. Increasing stability in ecological systems
derives largely from a greater redundancy of work‑gate functions and
from the potential that this redundancy offers for circumventing variable
energy flows within power circuits.
The greater redundancy
that exists within complex multispecies communities derives largely from
the role performed by competing species populations as "compensating
devices" (Whittaker and Woodwell 1972:151).
Interspecific competition serves to maintain community diversity,
because the conditions that eliminate one species from a forest community
may result in another species replacing it in the forest canopy, with the
larger community retaining existing levels of productivity, biomass and
functional diversity. Interspecific competition also reduces the probability
that closely related populations will exceed their resource supply,
because the size of a particular species population is unlikely to
increase significantly in the presence of numerous competing populations
(cf. Russo 1964; Hornocker 1970).
Predation also affects
species diversity. By
influencing prey population size, predation regulates interspecific
competition among prey species. Where
predators capable of preventing individual prey species from monopolizing
resources have either been missing or removed experimentally, the affected
communities have become less diverse (see Paine 1966).
Thus, while species diversity at lower trophic levels contributes
to species diversity in the higher trophic categories (through the flow of
energy in power circuits), species diversity at the higher trophic levels
can have a regulative impact on the size and diversity of species
populations in the lower trophic categories as well (through energy flow
in control circuits).
However, diversity by
itself does not enhance community stability.
Indeed, precisely the opposite may occur.
The key to maintaining community stability under variable
environmental conditions lies in the degree to which
redundancy
exists in the flow of energy/resources through a community. Only where redundancy exists can one population's response to
environmental variation be neutralized by the reaction of competing
populations, as well as by populations occupying distinct trophic levels.
Where insufficient redundancy exists, the negative consequences of
environmental fluctuations are likely to ramify throughout the community
and reduce community stability, even among communities containing high
diversity (see May 1973; Holling 1973; Leigh 1975).
Because the evolution of
endogenous rhythms requires a stable and predictable environment with the
consistent selective pressures that such conditions provide, the control
exerted by predators on the size and diversity of prey populations is
ultimately dependent on the reliability of the same prey species as
resources throughout the year. Thus, the enhanced community stability that
results from the regulative effect of community diversity derives
ultimately from the productivity and stability of the encompassing
ecosystem, because the complex regulative functions performed within
ecological communities require continuous and substantial resource flows
for their maintenance. Thus,
while capable of mitigating the numerous minor disturbances caused by
environmental instability, complex ecological communities are especially
vulnerable to major disruptions in the flow of energy.
These disruptions severely undermine the selective advantage of
specialization and, thus, jeopardize the niche differentiation upon which
the limited regulative capacity of such communities is based.
In summation, then, complex multispecies communities evolve as a result of the increasing specialization and niche differentiation generated by interspecific competition. Through the increasing intensification of resource exploitation, such communities evolve the most diverse species composition possible within the energetic limits of a particular environment. Because the selective advantage of specialization depends on a resource supply that is capable of supporting increasingly marginal adaptations, community diversity is determined by community productivity. At the same time, since diversity is ultimately a function of net productivity, maintenance costs impose a major constraint on community evolution. As a result, diverse ecological communities evolve in those ecosystems that support specialized adaptations and that reduce community maintenance costs. These conditions are best met in environments that are both productive and stable, that contain numerous, large and diverse habitats, and that are free from external exploitation. With increasing diversity, ecological communities evolve a greater internal regulation of energy flow and, thus, a limited independence from minor environmental fluctuations, provided resource flows within the community possess sufficient redundancy to compensate for local fluctuations in resource availability. However, the greater energy requirements needed to maintain complex ecological communities render these systems particularly vulnerable to major disruptions in their resource supply.
THE
EVOLUTION OF HUMAN COMMUNITIES
As with multispecies
communities, more complex human communities evolve largely due to the
opportunity costs (selective advantage) associated with greater
specialization under conditions of increasing community productivity and
population size. The
evolution of human communities is likewise determined by resource
availability, especially by those environmental conditions that present
either subsidies to or drains upon the developmental process.
Finally, more complex human communities also evolve endogenous
rhythms that facilitate their increasing independence from local
environmental variation.
Resource
Partitioning in Human Communities
As previously indicated,
the niche is a function that facilitates the flow of resources through an
ecological community. While
species diversity has most commonly been used to define the number of
distinct functions within multispecies communities,
occupational
categories and
functional
units have be employed to
determine the complexity of resource partitioning in human communities.
Because species, occupational categories and functional units all
effectively delineate the configuration of productive functions performed
within their respective communities, each represents an empirical variant
of an Operational Taxonomic
Unit (OTU) within niche
theory (see Vandermeer 1972). Each
varies in its specific dimensions as a result of the same competitive
process and in relation to resource availability (cf. Clark, et al. 1964).2
Occupational
categories may be defined in
terms of the type of activity performed together with the range of
resources processed and may include food production, food distribution,
building construction, mining, teaching, and so forth.
Each of these functions may, in turn, be divided into increasingly
restricted operations. Indeed, the increasing specialization of productive functions
is a central component of the evolution of complex human communities.
A
functional
unit may be defined as any
distinct organizational entity that participates in external exchange
relations and, thus, facilitates the flow of resources within a community.
In most recent Western communities, the functional unit has
normally been a business establishment (cf. Thomas 1960; Gibson and Reeves
1970; Smith 1976). However, functional units as diverse as a communal
village organization, a church, an irrigation company and a post office
operated among the early Little Colorado Mormon settlements considered
here. In order to understand
the evolution of complex human communities, it is important to distinguish
between "growth" and "development" (see Carneiro
1967). Growth refers simply
to an increase in the number of taxonomic units within a community,
whereas development denotes an increase in the kinds of units present.
Thus, while an increase in the number of farms in an agricultural
community constitutes growth, the emergence of new functional units and of
occupations other than farming represents development.
The evolution of complex human communities includes both growth and
development.
Occupations and
functional units (like species in multispecies communities) may be
arranged into a trophic hierarchy of producers and consumers.
This hierarchy is implied in the economic classification of
primary, secondary and tertiary industries, as well as in the distinction
made between basic and non-basic employment.
Within any community, some resource flows may be classified as
autotrophic
in that they generate the primary resources upon which the remainder of
the community depends. While
farming provided the basic community productivity among Little Colorado
Mormon settlements, both secondary and tertiary industries may serve as
the source of basic employment within a particular community, since local
communities may originated or evolve to exploit a variety of resources.
The Little Colorado Mormon towns, for example, have at various
times during the past century had economies that were based on farming,
ranching, lumber production, tourism and/or industrial production (see
Abruzzi 1985).
Heterotrophic
functions, on the other hand, distribute the net productivity provided by
autotrophic functions throughout the remainder of the community.
They may also perform work‑gate functions which regulate the
productivity of primary producers.6 Trophic levels are, of course, abstractions, and actual
functional units may operate on several trophic levels (see Ehrlich and
Birch 1964). Just as
phytoplankton in northern Sweden alternate seasonally between autotrophic
and heterotrophic functions (Rodhe 1955), so also may a food producing
unit (such as a farm) both produce and distribute the food that it grows.
Since the shifting of
resources from one productive activity to another involves specific costs,
individuals and functional units gain an adaptive advantage from
specialization: both competition and maintenance costs are reduced.
Thus, by increasing the efficiency of resource exploitation and,
therefore, the amount of
net
productivity available for
exchange, increased specialization enhances the aggregate flow of
resources through a community (see Samuelson 1958:653).
The effect that opportunity costs have on functional specialization
apply to substantively non-economic activities and functional units as
well. These must also compete
for the limited resources available within a community.
Other things being equal,
ecological theory suggests that an increase in community productivity
leads to an increase in population size within human communities, because
more resources exist upon which additional individuals can be supported.
Population growth, in turn, fosters an increase in the number and
diversity of occupations and functional units that derive their existence
from individual allocations of resources in productive activities.
Being opportunistic systems (at least with regard to resource
exploitation, functional specialization and community diversification)
human communities, like other ecological systems, evolve to the
organizational limits imposed by available resources.
Similarly, mutual causality operates in the evolution of human
communities as well. While
occupational and functional unit specialization and differentiation
contribute to increasing community diversity, existing productive and
distributive arrangements select for the viability of specific
additional activities within a community, as well as for whole new avenues
of community development. Moreover,
because specific occupations and functional units require distinct
population and resource thresholds in order to exist within a community,
various functions are added to human communities at different rates during
the course of community development (cf. Thomas 1960; Carneiro 1962,
1968; Haggett 1966; Gibson and Reeves 1970).
An important distinction
exists between human and nonhuman ecological communities with regard to
the relationship between productivity and populations size.
Although many human communities may, like other ecological
communities, evolve in response to initial increases in productivity, more
often it would appear the evolution of complex human communities occurs in
response to the adaptive pressures resulting from population growth within
a fixed habitat (cf. Boserup 1965; Wilkinson 1973; Cohen 1977; Simon 1977;
Abruzzi 1979, 1980; Sanders and Nichols 1988).
An increase in population size stimulates increases in community
productivity and functional diversity by increasing both the supply of and
the demand for increased resource availability within a community. However, permanent increases in population size can only
occur in conjunction with concurrent increases in community productivity.
Consequently, population increase within a circumscribed habitat
requires an additional intensification of resource exploitation in order
to raise the aggregate productivity of a given territory.
Such pressure for the intensification of resource exploitation
places a premium on the specialization of community functions due to the
more effective resource exploitation and the enhanced net productivity
that such specialization provides. Finally,
population growth within a fixed habitat demands an increase in per capita
energy flows (cf. Boserup 1965:41‑55; Harris 1977:176, passim),
which increases aggregate community productivity even further.
Continued population
growth within a fixed habitat also selects for the evolution of regulative
functions that assure sufficient and stable levels of productivity.
Consequently, while population growth generates a greater number
and diversity of functional units through its effect on productivity, it
also stimulates the diversification of functional activities and
organizations that serve as control circuits directing increasing
resources into channels expanding community productivity due to the
increased demand for resources that such growth creates.
Thus, whether specific
human communities evolve in response to initial increases in productivity
or population growth, the basis of community evolution remains the same.
The selective advantage of specialization and niche differentiation in
either case derives from the opportunity costs associated with resource
partitioning in the presence of an expanded flow of resources.
In both situations, the degree to which functional specialization
proceeds depends upon the ability of individuals to subsist on
increasingly narrow and more marginal resource flows.
Community diversity, thus, remains a function of the aggregate flow
of resources in a community. However,
the enhanced positive feedback that exists between productivity,
population growth and community diversity in human communities does not
undermine the applicability of the ecological model to these communities.
The population increase that accompanies the evolution of complex
human communities is founded on a simultaneous increase in community
productivity made possible through the evolution of control circuits
circumventing environmental limitations.
As predicted by ecological theory, increasing community diversity
within human communities evolves as a function of concurrent increases in
community productivity and population size within specific limits imposed
by local and regional environmental conditions.
Subsidies
and Drains in Human Communities
As with all ecological
systems, the maintenance and survival of human communities depends
ultimately on the availability of resources.
Thus, the various external conditions that effect human resource
exploitation may also be viewed within an
energy
subsidy/energy drain
perspective. Similarly,
phenomena that provide energy subsidies under one set of circumstances may
impose energy drains under different circumstances, even within the same
community. In addition, the
rate at which conditions impose themselves relative to the adaptive
capacity of local populations is as important a feature of the
subsidy/drain dichotomy in human communities as it is in nonhuman ones.
While rainfall and a permanent stream generally provide relatively
cheap energy inputs (subsidies) into agricultural productivity, excess
rainfall and flooding rivers can impose a severe drain that either reduces
agricultural production or increases the cost of achieving the same level
of productivity. Furthermore, just as different amounts of precipitation and
streamflow can have distinct effects on the maintenance costs associated
with irrigation and agricultural productivity within a farming community,
so also can distinct conditions of population growth have different
effects on community development. While
those conditions which promote stable population growth actually stimulate
the evolution of more complex human communities (Boserup 1965; Culbertson
1971; Wilkinson 1973; Simon 1977), those which yield sudden increases in
the size of a population (most notably through rapid immigration) may
impose a severe drain on community development by increasing the stress on
local resources and leading to a greater proportion of productive
resources having to be channeled into strictly maintenance functions (cf.
Abruzzi 1993:31).3
Productivity
and Stability in Human Communities
While large discrepancies
between productivity and biomass are unlikely to occur among nonhuman
communities, substantial differences in per capita productivity and
standard of living occur quite frequently among human communities.
This difference complicates the relationship between community
productivity, population size and functional diversity in human
communities (see Culbertson 1971:35-101; Wilkinson 1973). Per capita productivity
must, therefore, be included as a necessary supplement to aggregate
productivity inhuman communities in order to more accurately represent the
surplus resources (net
productivity) available to
maintain community diversity in these communities.
The evolution of complex human communities, with their enhanced
differentiation, interdependence, organization and managerial functions,
demands an expensive allocation of community resources and, thus, depends
fundamentally on increases in per capita productivity (see Harris 1959,
1980:183-206; H. Odum 1971; Simon 1977). As
a result, those factors that reduce per capita productivity inhibit
community evolution. For the Little Colorado Mormon settlements, those specific
conditions that limited agricultural productivity or that increased the
size of the investment required to sustain existing levels of productivity
reduced available net productivity and, thus, inhibited community
evolution.
The same factors that
limit specialization in those human communities located in unproductive
environments operate in communities situated in unstable ones as well.
Moreover, differences in the amplitude, frequency and
predictability of environmental fluctuations have distinct effects on the
development of human communities too.
Differences in both
the amount of resources required to rebuild dams and the frequency of dam
reconstructions yielded a disproportionate drain upon the various Little
Colorado Mormon towns. One of
the critical factors influencing local community development was the
degree to which environmental variation could be anticipated and
controlled. Where the
principal limiting factor was a variable and unpredictable growing season,
as was the case at higher elevations, little anticipation or control could
be exerted. Where, on the
other hand, agricultural productivity was limited by seasonal variation in
surface water availability, a measure of anticipation and control could be
gained through the construction of storage reservoirs, provided suitable
dam sites were available.
Habitat
Size and Diversity
Habitat
size is
directly related to community evolution.
The amount of economically exploitable farmland, for example,
directly affects the potential aggregate productivity, per capita
productivity, population size and functional diversity of an individual
agricultural settlement. Habitat diversity
also facilitates the evolution of complex human communities, because
different portions of habitat may exhibit distinct conditions of resource
availability. Habitat
diversity is also likely to be at least partially a function of habitat
size.
Exploitation
As previously indicated,
exploitation occurs whenever resources that may be used to increase
population, productivity or stability of one community are expropriated
from that community in order to enhance the development of another system.
Exploitation is a common feature of the exchange that takes place
between ecological systems of unequal complexity, and more complex
communities generally exploit the less complex systems around them (Margalef
1968). Expanding frontiers
between contiguous ecological communities results largely from the
competitive advantage that more complex communities possess in relation to
the less complex systems on their periphery.
The expansion of the American frontier was no different (cf.
Shannon 1945). As this
frontier expanded into the Little Colorado River Basin, specific resources
that could have contributed to the development of these indigenous
communities were expropriated from local use.
This loss of exploitable resources imposed a substantial drain on
the indigenous Mormon population and seriously threatened the success of
their colonization effort (see Abruzzi 1993:165-191, 1994).
Regulation
within Human Communities
The evolution of complex
human communities has invariably been characterized by an increase in the
number and specificity of regulative functions (i.e., control circuits).
Two general kinds of control circuits may be distinguished in human
communities:
indirect
(consumer) and
direct
(management) regulative
functions. The former include
those functions and functional units which, through their effect on the
demand for specific resources, regulate the output of a community's
producers. Consumer functions
affect the opportunity costs associated with specific resource allocations
among competing producers, and the proportion of consumer functions
providing feedback into productivity increases with the evolution of more
complex human communities.4
Of greater significance
to the evolution of complex human communities has been the increased
control exerted by direct regulative functions. More complex human
communities possess a larger proportion of management functions to total
community organization than do less complex systems, and direct regulative
functions have evolved historically to control an increasing share of
community resources. Although
governmental functional units have performed the principal management
functions in communities since the emergence of the state, critical
management functions may be performed by functional units other than those
under governmental administration. Among
the early Mormon settlements in the Little Colorado River Basin, the local
church organization and its affiliated institutions performed many of the
management functions needed to facilitate community development (see
Abruzzi 1989; 1993:143-163, 180-181).
The ecological model
suggests that more complex human communities possess a greater capacity
for responding to environmental disturbances than do less complex
communities, and that the former systems are more likely to achieve the
endogenous regulation of community parameters.
Having achieved a greater independence from local habitat
variability, more complex human communities possess a selective advantage
in adapting to unstable environmental conditions.
As with non‑human ecological communities, however, it is the
greater
redundancy of resource
flows that enables complex human communities to achieve their greater
stability. Where a community
depends disproportionately upon a single resource, any variation in the
availability of that resource will ramify throughout the community.
Increasing the number and diversity of distinct local environments
that are integrated into a single system of resource redistribution, on
the other hand, enhances the adaptive capacity of a complex human
community because it increases the number of functionally independent
resource flows available to compensate for local productive deficiencies
(cf. Coe and Flannery 1964; Sanders and Price 1968).
However, the regulative capacity of human communities must also be
viewed hierarchically. Complex human communities can only offset deficiencies in
local production to the extent that aggregate environmental conditions are
productive and stable enough to maintain the specialized functions which
underlie resource redistribution (see Abruzzi 1982:18; 1987).
In summation, then, the
extension of ecological theory to human communities suggests that these
communities, like their non-human counterparts, evolve as a result of
resource partitioning among potential competitors.
Due to the non-Malthusian
basis of human population ecology, however, human communities can
substantially enhance the level of population, productivity and functional
diversity achieved within a particular community by intensifying resource
exploitation well beyond that possible in nonhuman communities.
However, the potential for positive feedback that exists between
population, productivity and functional diversity in human communities
does not contradict the general ecological model; rather, human
communities represent a special case operating in accordance with the
general principles prescribed by that model.
Continued increases in population size and community diversity
depend fundamentally on increases in the abundance and reliability of
community productivity. Moreover,
the evolution of human communities is subject to the same environmental
constraints that limit community productivity and stability and that
affect the cost of maintaining community operations in nonhuman
communities. Similarly, while
more complex human and nonhuman communities both posses an adaptive
advantage due to their greater capacity for limited self‑regulation,
endogenous rhythms in both types of systems depend on a redundancy of
resource flows within them. Consequently,
like their nonhuman counterparts, the organization of complex human
communities is highly vulnerable to major disruptions in energy flow.
If the ecological model of community development outlined here is to be successfully applied to Mormon settlements in the Little Colorado River Basin, developments accompanying the settlement process must conform to expectations derived from that model. Those settlements that were located in the most productive and stable environments and that experienced the lowest maintenance costs associated with agricultural production should have achieved the greatest aggregate productivity, per capita productivity, population size, and community stability. These same settlements should also have been the most functionally diverse. Conversely, the least functionally diverse settlements should have displayed the lowest aggregate productivity, per capita productivity and population size. They should also have been located in the least productive and most unstable habitats, as well as those that imposed the highest maintenance costs associated with agricultural production. Finally, to the extent that the redistribution of resources among individual settlements enhanced the success of the colonization effort, it should have been based on the integration of resource flows from numerous independent habitats experiencing distinct schedules of environmental variation. Only then could resource redistribution possess the redundancy needed for effective environmental regulation.
MORMON
COLONIZATION OF LITTLE COLORADO RIVER BASIN
Mormon colonization of the Little Colorado River Basin began in 1876 when some 500 Mormon pioneers established four agricultural settlements --Sunset, Brigham City, St. Joseph and Obed-- along the lower valley of the Little Colorado River (see Figure 2). These initial settlements served as bases for the founding of some two dozen additional colonies throughout the river basin, including Woodruff, St. Johns and Eagar along the upper Little Colorado River, Snowflake and Taylor on Silver Creek, and Showlow and Alpine in the southern highlands. However, despite a considerable investment of manpower, a high degree of cooperation among local communities and continuous material support from Church headquarters in Salt Lake City, the Little Colorado colonies experienced considerable local variation in agricultural production. St. Joseph suffered complete crop failures during three of the seven years between 1876 and 1882. Sunset produced an abundant harvest in 1879, but had to be abandoned in 1883 following three years of poor harvests. Brigham City failed to produce even one successful harvest and was finally abandoned in 1881. In addition, records indicate that either poor harvests or complete crop failures prevailed throughout the river basin during half the years between 1880 and 1900. In the end, while Snowflake, Taylor, Eagar and St. Johns grew to several hundred inhabitants, produced relatively abundant and reliable harvests and contained a diversity of occupations and businesses, Woodruff, St. Joseph, Showlow and Alpine contained less than one hundred persons each and were substantially less productive and diverse. In fact, these latter towns barely survived.
Figure 2
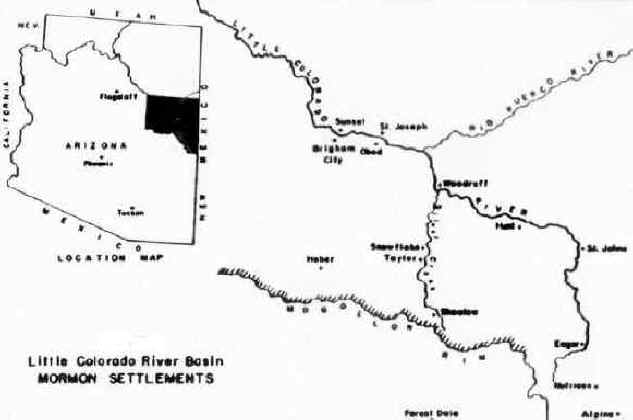
The principal factor
influencing community development among the Little Colorado Mormon
settlements was the nature of the physical environment to which the
farmers in these towns had to adapt.
The Little Colorado River Basin encompasses some 5,000 square miles
and increases in elevation from about 5,000 feet in the lower valley of
the Little Colorado River southward to about 8,500 feet along the Mogollon
Rim, a steep escarpment that defines the southern boundary over much of
the region. In addition,
several mountain peaks exceeding 10,000 feet exist in the eastern portion
of the southern highlands. Climate
throughout the region is arid to semi-arid, with annual precipitation
ranging from 9 inches at lower elevations in the north to almost 25 inches
in the southern highlands. As
a result, northern desert vegetation predominates in the lower valley of
the Little Colorado and is succeeded southward by grassland,
juniper-piñon woodland and montane forest communities.
Since most of the basin receives less than 15 inches annual
precipitation, the grassland and juniper-piñon woodland communities cover
nearly 80% of the total surface area.
In addition, bare soil accounts for between 55-65% of the total
surface cover within the grassland community (Dames and Moore 1973[section
4]:201).
In contrast to
precipitation, length of the growing season varies inversely with
elevation and ranges from an average of 87 days near Alpine to 179 days at
St. Joseph. Thus, both the
length and the reliability of the growing season vary inversely with
average annual precipitation, restricting dependable agriculture to river
valleys at lower elevations. Early
pioneers also had to contend regularly with early frosts, high
temperatures, droughts, flooding, hailstorms, insects and high winds.
Finally, two devastating droughts ravaged the basin for nine years
between 1892-1905, killing thousands of livestock and causing widespread
crop failure. Such pervasive
environmental variation frequently resulted in the same settlement losing
crops to several causes during a single agricultural season (cf. Abruzzi
1993:23-25).
The most important
environmental factor influencing community development in this arid river
basin has been the availability of suitable water for irrigation (see
Abruzzi 1985). The unreliability of precipitation made all early farming
settlements in the region necessarily dependent on surface water for
irrigation. However, since
streams throughout the region flow primarily in direct response to
precipitation and ambient temperature, surface water availability follows
a distinct annual cycle. Runoff
is generally moderate between January and March due to the melting of
snowpacks at higher elevations and declines as these snowpacks disappear.
Except for streams at higher elevations, most streambeds throughout
the basin are dry from April to June when 45% of annual irrigation
requirements must be applied (see Bureau of Reclamation 1947:72). Streamflow increases dramatically following the onset of
intense summer storms in July and subsides as these summer storms pass.
It then remains low until snow re-accumulates at higher elevations.
Although stream-flow
variability is widespread, it is greatest in the lower valley of the
Little Colorado River where the largest surface area is drained.
Since no suitable reservoir sites exist at lower elevations, the
lower valley settlements could only construct diversion dams. These settlements, therefore, remained completely vulnerable
to the greatest stream-flow variability in the basin. Variation in streamflow also yielded a higher incidence of
dam failures among the lower valley towns than anywhere else in the basin.
St. Joseph and Woodruff suffered 13 and 10 dam failures
respectively between 1876 and 1900, compared with only two at St. Johns,
three at Snowflake and Taylor, one at Showlow, and none at Eagar and
Alpine.
The direct costs imposed
by dam failures and the subsequent flooding of fields included not only
the time, materials and manpower required to rebuild the dams themselves,
but also those needed to repair ditches and replant fields.
The indirect costs of dam failures included the labor that could
not be invested in other productive activities, as well as the detrimental
effect that repeated flooding had on soil fertility.
The fact that only two of the six towns established in the lower
valley survived strongly suggests that the cost of farming was highest in
this portion of the basin.5
No other section lost as many settlements.
Furthermore, the history of dam failures at St. Joseph and
Woodruff, the only two lower valley settlements to survive, demonstrates
clearly that these towns would also have failed had it not been for the
repeated subsidies of food, supplies and labor they received from the
other Mormon towns in the region, as well as from Church sources outside
the basin (see Abruzzi 1989, 1993:123-131).
Local
Differences in Community Development
For purposes of understanding local differences in community development, the Little Colorado River Basin may be conveniently divided into three subregions: (1) the lower valley of the Little Colorado River; (2) the southern highlands; and (3) the intermediate territories. The least developed of all the communities studied were those, such as Showlow and Alpine, that were located in the southern highlands. Although annual precipitation was highest in this subregion, the growing season there was both the shortest and the least reliable, considerably less than the 120 days needed for most crops. Furthermore, even though soils are deeper at higher elevations due to the greater density of vegetation in this subregion, they tend to be poorly drained, susceptible to flooding and, in many places, slightly acidic. In addition, mountain valleys tend to be small and, thus, not very conducive to the local expansion of agriculture. Communities in the southern highlands, therefore, achieved: (1) the smallest populations, (2) the lowest and most variable agricultural productivities and, consequently, (3) the least number and variety of occupations and businesses (see Table 1). They also contained the least developed Church organization (Abruzzi 1993:43). By any measure of community development, the southern highlands settlements were the least developed Mormon towns in the region. Stated in ecological terms: low environmental productivity and stability resulted in a low and highly variable aggregate and net community productivity among southern highland settlements. As predicted by ecological theory, these settlements were the least functionally diverse Mormon towns in the basin.6
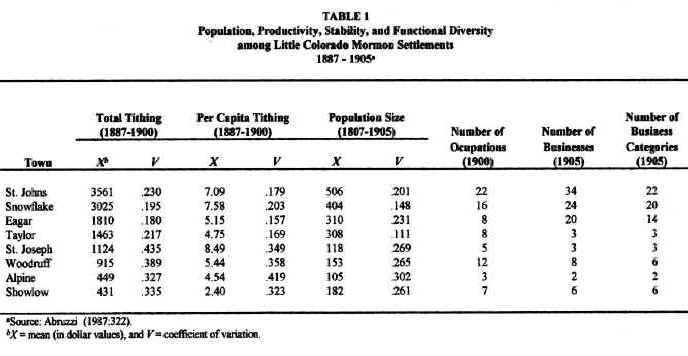
Settlements along the lower valley of the Little Colorado River,
such as St. Joseph and Woodruff, enjoyed more than ample growing seasons. They, therefore, possessed the potential for supporting
larger populations and achieving substantially greater productivity and
functional diversity than settlements in the southern highlands. However, the lower valley settlements experienced high summer
temperatures, frequent dust storms and a recurring spring dry season
that combined to reduce agricultural productivity and increase the
frequency of crop failures. Lower
valley settlements also had to contend with poor quality soils that are
high in sodium and low in both phosphorus and organic matter.
In addition, due to their high clay composition, these soils
possess low permeability and are highly susceptible to flooding when
irrigated. The lower valley settlements also had to irrigate their
relatively infertile soils with the poorest quality surface water in the
region. Although the Little
Colorado River originates as a clear mountain stream in the southern
highlands, by the time it reaches the lower valley it has received
considerable runoff throughout the grassland community, and its sediment
load approaches 20% of streamflow (Bureau of Reclamation 1950:3, 10). Furthermore, both soil and water quality deteriorated
steadily throughout the lower valley during the nineteenth century due to
extensive overgrazing throughout the grassland community and to the prior
appropriation of surface water upstream on both the Little Colorado River
and Silver Creek (see Abruzzi 1994).
As already indicated, the
lower valley settlements also suffered significantly more dam failures
than any of the other Little Colorado Mormon towns, making farming in this
subregion more difficult and more costly than anywhere else in the basin.
Since the lower valley settlements could build only diversion dams,
they also remained completely vulnerable to the intense variability
displayed by the Little Colorado River at lower elevations.
These environmental limitations combined to make the lower valley
settlements moderately more productive, but only slightly larger than
those in the southern highlands (see
Table 1).
With the highest incidence of dam failures occurring among some of
the smallest populations, lower valley settlements also bore among the
highest per capita maintenance costs in the region.
Indeed, so great were the maintenance costs relative to
productivity among lower valley settlements, that not only did these towns
not contain substantially more occupations or businesses than those in the
southern highlands, but only two of the six Mormon towns established in
this subregion even survived.
In
ecological terms, the lower valley settlements were situated in highly
unstable habitats which possessed only moderate productivity, but which
imposed especially high community maintenance costs.
Moderate environmental productivity in the face of low
environmental stability and high maintenance costs yielded only limited
aggregate and net community productivity for these towns.
As predicted by ecological theory, lower valley settlements
contained among the smallest and most variable populations in the region
and achieved a functional diversity that was not appreciably greater than
that found among settlements in the southern highlands.
Significantly, the negative effect that environmental instability
had on community diversity among lower valley settlements relative to
those in the southern highlands is comparable to the effect that Sanders
(1968) indicates environmental variability had on the relative diversity
of tropical versus temperate benthic communities.
In both cases, the developmental benefits of greater productivity
were negated by reduced stability.
The most successful local Mormon settlements were Snowflake, Taylor, St. Johns and Eagar, all of which were located in river valleys at intermediate elevations. These towns all enjoyed adequate and reliable growing seasons (over 120 days per year) and were located near permanent streams whose relatively abundance and reliability were enhanced through the construction of storage reservoirs. The access of intermediate settlements to dependable water supplies and adequate growing seasons made them less vulnerable to the negative effects of climatic variability than towns located either along the lower valley of the Little Colorado River or throughout the southern highlands. Each of the intermediate towns was also located in relatively large valleys containing fertile and well-drained soils. They thus achieved the largest agricultural productivities, populations and number and diversity of occupations and businesses of any towns in the region (see Table 1). Moreover, two of these towns --Snowflake and St. Johns-- evolved the most complex Church organizations, including the two regional stake organizations that coordinated the religious and temporal affairs of all Mormon towns in the basin.7
From
the perspective of general ecology, large valleys, good soils and
abundant, superior quality surface water translated into high
environmental productivity for intermediate settlements.
At the same time, reliable growing seasons, together with stable
surface water sources, provided high environmental stability with regard
to critical agricultural resources. High environmental productivity and
stability combined to produce the highest and least variable community
productivities of any settlements in the region.
Furthermore, because intermediate settlements did not suffer the
frequency of dam failures experienced in the lower valley; and because
they contained the largest populations with which to undertake dam
reconstruction, they also sustained the lowest per capita maintenance
costs in the region. They, therefore, generated the highest net productivities and
were able to support the largest and most stable populations in the basin.
As predicted by ecological theory, intermediate settlements evolved
a greater functional community diversity than any other Mormon settlements
in the region.
Ecological
theory not only explains sub-regional differences in community
development; it also provides an explanation for the specific variation in
community development displayed by individual Mormon settlements in the
region. A
Rank-Order
Correlation of .884 (p<.01)
was achieved when individual Mormon settlements were compared for
composite indices of population size, community productivity and
community stability on the one hand and functional community diversity on
the other (see Table 2).
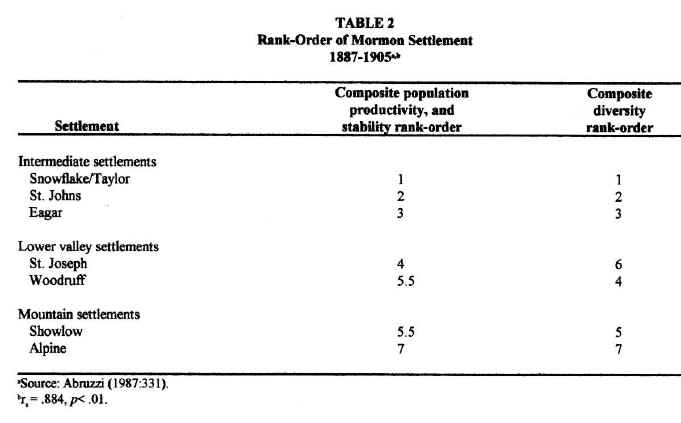
a Because
Snowflake and Taylor are located within three miles of each other, they shared a common irrigation system and contained businesses
that served both towns. They are, therefore, treated as a single
community in the calculations performed (see Abruzzi 1993:194-195).
Resource
Redistribution and Successful Mormon Colonization
Successful
colonization of the Little Colorado River Basin was due in large part to
the development of a system of resource redistribution that mitigated the
negative consequences of local environmental variability (see Leone 1979;
Abruzzi 1989, 1993:148-155). The region's pronounced spatial diversity
provided early settlers with a unique opportunity to overcome local
environmental limitations. Due
largely to local differences in topography and precipitation, the basin
contains numerous, widely separated local habitats that were often
differentially affected by the same regional environmental influences and
which, therefore, offered distinct potentials for agricultural
productivity (see Abruzzi 1989). A
clear adaptive advantage, thus, existed for these early settlements to
integrate the productivities of their diverse habitats into a single
multi-habitat resource redistribution system.
Resource redistribution among individual settlements would have
enabled each town to effectively diversify its resource base and, thus,
increase its ability to circumvent local environmental variability.
The Little Colorado Mormon settlements developed two distinct multi‑habitat
resource redistribution systems during the nineteenth century. The first consisted of several productive enterprises,
including a sawmill, a dairy, a tannery and a grist mill, jointly operated
by the initial settlements established in the lower valley of the Little
Colorado River (see Abruzzi, 1993:143-147).
These enterprises were generally located at higher elevations to
the south and provided the lower valley settlements with important
resources (most notably lumber, cheese, butter, meat and certain
vegetables) that could not be produced near the towns themselves.
They, thus, supplemented the highly variable farming productivity
achieved within the lower Little Colorado River Valley.
However,
the conjoint enterprises were largely summer operations in the southern
highlands which, due to their distance from the lower valley, required a
more or less permanent resident population.
These enterprises, thus, competed directly with farming in the
lower valley for labor. As
population size declined and as the number of settlements in the lower
valley decreased under the strain of recurring dam failures, the conjoint
enterprises could no longer be maintained and were eventually abandoned.
A
second system of multi-habitat resource redistribution emerged following
the failure of the conjoint enterprises.
This latter system operated through the redistribution of locally
collected tithing resources. Tithing,
was mostly paid in kind to local church leaders and forwarded to regional
Church warehouses in Snowflake and St. Johns where it was stored and
redistributed to those in need (see Leone 1979:43-85; Abruzzi
1993:148-155). Through tithing redistribution, individuals, and sometimes
entire towns, were able to acquire needed resources produced elsewhere in
the basin. Through the
redistribution of tithing resources, these early Mormon settlers used the
region's spatial diversity to offset its local temporal variability.
In the process, they transformed a potentially dormant surplus into
a flow of resources that was ultimately responsible for the success of the
colonization effort. St.
Joseph and Woodruff would clearly never have survived their numerous dam
failures had it not been for tithing redistribution.
They would likely have become extinct, as had every other
settlement in the lower valley.
The system of
multi-habitat exploitation based on the conjoint enterprises was not
ecologically viable, because it was incompatible with local and regional
environmental conditions. The small populations in the lower valley suffered intense
drains on their limited resources due to the intense variability of
surface water in the Little Colorado River.
Flooded fields and repeated dam failures imposed a chronic labor
shortage throughout the lower valley settlements and severely limited
their ability to exploit distant habitats.
This system thus failed as a mechanism of environmental regulation.
Tithing
redistribution, on the other hand, succeeded as a mechanism of
environmental regulation for the very reasons that the conjoint
enterprises failed. It was
able to circumvent local environmental variation in a way that the system
of conjoint enterprises could not. Because
it included every Little Colorado Mormon town and, therefore, integrated
resource flows from every exploited habitat, the system of tithing
redistribution was able to direct a substantial flow of resources at the
specific times and places that resources were critically needed to
counteract the destabilizing impact of environmental variation.
In addition, tithing redistribution integrated the productivity and
labor of over 2,000 persons in some two dozen separate settlements located
within as many functionally independent habitats scattered throughout
the entire river basin. The
conjoint enterprises, on the other hand, integrated the material resources
of only a few hundred persons inhabiting three or four neighboring
settlements located in contiguous, highly unstable habitats, all of which
experienced the same schedule of variation --including the timing of dam
failures. The effectiveness
of tithing redistribution was also substantially enhanced by the
integration of local settlements into a regional, centrally administered
religious organization and by the affiliation of its primary
institutions with parent organizations outside the region (see Abruzzi
1989; 1993:150-157). While
the former increased the responsiveness and reliability of local tithing
redistribution, the latter provided access to resources whose availability
was completely independent of local and regional environmental
conditions.
The
differential success of Mormon attempts at multi‑habitat
exploitation in the Little Colorado River Basin likewise conforms with
expectations derived from general ecological theory.
Ecological redundancy was clearly absent in the system of conjoint
enterprises established by the early Mormon settlements in the lower
valley of the Little Colorado River.
Although these enterprises exploited resources away from the lower
valley, the availability of the labor needed to operate them was directly
linked to the material conditions affecting agriculture along the Little
Colorado. As a result, the
negative effect that environmental instability had upon farming in the
lower valley ramified to the conjoint enterprises and caused their demise.
The system of conjoint enterprises, thus, failed as a mechanism of
environmental regulation despite its cooperative orientation, its
communal organization and its explicit ethno-ecological foundation.
The
system of tithing redistribution, on the other hand, integrated
settlements throughout the entire river basin by uniting the total
productivity and labor of some two dozen separate populations concentrated
in the intensive and independent exploitation of a variety of distinct
local habitats. Productive shortfalls at one location were generally
compensated for by surplus productivity elsewhere in the region.
Thus, separate resource flows originating in numerous independent
habitats provided the specific redundancy needed to offset local habitat
variability. Linking local
tithing redistribution to encompassing Church institutions simply enhanced
the redundancy already present in this system of resource redistribution.
Tithing redistribution,, thus, succeeded as a mechanism of environmental
regulation despite the fact that it was based on individual profit and
that its primary institutions were established for expressly
non-ecological purposes (see Abruzzi 1989).
CONCLUSION
I have argued
here that the general model of community development used to explain the
evolution of complex ecological communities provides a useful model for
the evolution of complex human communities as well.
I have proposed that the applicability of the general ecological
model to human communities rests on the fact that human communities are
ecological communities, and that the model in question applies to all
ecological communities regardless of their specific biological
composition. I have then
applied the ecological model to explain specific historical developments
associated with Mormon colonization of the Little Colorado River Basin.
Specifically, I have used the model to account for:
(1)
local and subregional differences in community development,
(2)
the role of resource redistribution in successful colonization, and
(3)
the variable success of Mormon efforts to develop a viable resource
redistribution system.
Ecological
theory successfully explains each of these historical developments.
It thus provides a more precise and parsimonious explanation of
this settlement process than all previous accounts (compare, for example,
Peterson 1973 and Leone 1979 with Abruzzi 1993).
Moreover, it does so within an objectively verifiable explanatory
framework. The relative
success of the ecological model compared to earlier studies of Mormon
colonization in the Little Colorado River Basin is due in large part to
its application as a set of general principles from which specific local
historical developments are systematically explained, rather than simply
as a metaphor orienting the study of human-environmental relations in this
region. The application of
ecological theory to Mormon colonization of the Little Colorado River
Basin suggests, therefore, that ecological theory should
be applied more (not less) strictly in the study of human ecology.
It is only through the explicit application of ecological theory to
human populations that the validity of such applications can be
objectively evaluated, and that human ecology can make a meaningful
contribution to the development of ecological theory.
NOTES
1. The term regulation refers here simply to the existence of
processes through which the activity of one population influences the
abundance and distribution of resources that affect the existence of other
populations in a community. No
equilibrium is implied.
2.
Resource distribution
in both human and nonhuman communities actually results from the
activities of individual organisms participating in a variety of
independent exchange relations. Species and comparable functional
categories in human communities comprise equivalent Operational Taxonomic
Units from the perspective of niche theory in that both delineate the
channeling of energy flow in their respective communities. The precise
calculation of all individual productive activities would provide the
optimum basis for examining functional diversity and resource partitioning
in ecological communities. However,
such data is unavailable for most communities. Consequently, more general
categories must be used.
3.
By consuming resources which could otherwise be invested in productive
activities, rapid population growth reduces the flow of net productivity
available to support increasingly specialized adaptations. From this
perspective, overpopulation may
be defined as any excess of population over productivity that reduces per
capita resource availability and, thus, threatens the survival of existing
organisms in the community. Such overpopulation contributes to community
simplification because more marginal niches become less viable as
pressures mount to channel an increasing proportion of a community's
resources into maintenance functions and to broaden the range of resources
exploited by individuals and by specific functional units.
4.
Anthropologists have illustrated how indirect consumer functions such as
the numaym and potlatch among the Kwakiutl of the American Northwest (cf.
Piddocke 1965) and the "big man" and reciprocal feasting among
Kaoka-speaking peoples of Guadalcanal (Hogbin 1964) enhanced local
community productivity. In
much the same way, a successful restaurant or restaurant chain may
stimulate the production of specific food resources within a contemporary
Western community.
5.
Obed was abandoned in 1877 for health reasons.
Also, another town, known as "Old Taylor", was located
about 6 miles downstream from St. Joseph.
This town was founded and abandoned in 1878 following the failure
of 5 dams.
6.
Significantly, Alpine
and nearby Nutrioso, the two most highly-situated settlements in the
region, registered the highest proportion of non-heads of households
declaring farming as their principal occupation on the 1900 census. The fact that many of these individuals were between 10-15
years of age suggests that intense demands were imposed upon farming at
this altitude, resulting in the routine application of all available labor
(see Abruzzi 1993:119-120).
7.
The Mormon Church is administratively divided into stakes and wards, which
may be compared to diocese and parishes respectively in the Roman Catholic
Church. During the nineteenth century, each Little Colorado Mormon
settlement comprised one ward. Wards
along the lower valley of the Little Colorado River were initially
organized into the Little Colorado Stake, with the remaining wards were
included in the Eastern Arizona Stake.
In 1887, the Little Colorado wards were reorganized into the
Snowflake Stake and the St. Johns Stake.
These two stake organizations contained the western and eastern
wards respectively.
REFERENCES CITED
Abruzzi,
W. S. 1979. Population Pressure and Subsistence Strategies among the Mbuti
Pygmies. Human Ecology 7:
183-189.
-----.
1980. Flux among the Mbuti Pygmies of the Ituri Forest: An Ecological
Interpretation. In Beyond the
Myths of Culture: Explorations in Cultural Materialism, edited by E.
Ross, pp. 3‑31. New York: Academic Press.
-----.
1982. Ecological Theory and Ethnic Differentiation Among Human
Populations. Current Anthropology 23:13-35.
-----.
1985. Water and Community Development in the Little Colorado River Basin. Human Ecology 13:241-269.
-----.
1987. Ecological Stability and Community Diversity during Mormon
Colonization of the Little Colorado River Basin. Human
Ecology 15:317-338.
-----.
1989. Ecology, Resource Redistribution, and Mormon Settlement in
Northeastern Arizona. American
Anthropologist 91:642-655.
-----.
1993. Dam That River! Ecology and
Mormon Settlement in the Little Colorado River Basin. Lanham, MD:
University Press of America.
-----. 1994. The Social and Ecological Consequences of Early Cattle Ranching in
the Little Colorado River Basin. Human
Ecology (in press).
Adams,
R. N. 1975. Energy and Structure: A
Theory of Social Power. Austin: University of Texas Press.
Alexander,
R.D., and G. Borgia. 1978. Group Selection, Altruism, and the Levels of
Organization of Life. Annual Review
of Ecology and Systematics 9:449-474.
Ashby,
W.R. 1956. An Introduction to
Cybernetics. London: Chapman and Hall.
Athens,
J.S. 1977. Theory Building and the Study of Evolutionary Processes in
Complex Societies. In For Theory
Building in Archaeology. edited by L.R. Binford, pp.353-384. New York:
Academic Press.
Barth,
F. 1956. Ecological Relationships of Ethnic Groups in Swat, North
Pakistan. American Anthropologist 58:1079-1089.
-----.
1967. On the Study of Social Change. American
Anthropologist 69:661-669.
Bennett,
J.W. 1969. Northern Plainsmen:
Adaptive Strategy and Agrarian Life. Chicago: Aldine-Atherton.
-----.
1976. The Ecological Transition:
Cultural Anthropology and Human Adaptation. New York: Pergamon.
Blau,
P. 1967. Exchange and Power in
Social Life. New York: Wiley.
Boserup,
E. 1965. The Conditions of
Agricultural Growth: The Economics of Agrarian Change under Population
Pressure. Chicago: Aldine.
Brodbeck,
M. 1962. Explanation, Prediction, and "Imperfect" Knowledge. In Scientific
Explanation, Space and Time. H. Fiegl and G. Maxwell, eds.
Minneapolis: University of Minnesota Press.
Brookhaven
National Laboratory. 1969. Diversity and Stability in Ecological Systems. Brookhaven
Symposia in Biology No. 22. Springfield: U.S. Department of Commerce.
Brown,
L.L., and E.O. Wilson. 1956. Character Displacement. Systematic
Zoology 5:49-64.
Bureau
of Reclamation. 1947. Snowflake
Project Arizona. Project Planning Report 3-8b.2-0. Washington, D.C.:
U.S. Department of the Interior.
Carniero,
R.L. 1962. Scale Analysis as an Instrument for the Study of Cultural
Evolution. Southwestern Journal of
Anthropology 18:149-169.
-----.
1967. On the Relationship between Size of Population and Complexity of
Social Organization. Southwestern
Journal of Anthropology 23:234-243.
-----.
1968. Ascertaining, Testing and Interpreting Sequences of Cultural
Development. Southwestern Journal of
Anthropology 24:354-374.
-----.
1970. A Theory of the Origin of the
State. Science 169:733-738.
Cartmill,
M. 1994. Reinventing Anthropology: American Association of Physical
Anthropologists Annual Luncheon Address, April 1, 1994. Yearbook
of Physical Anthropology 37:1-9.
Catton,
W.R. 1993. Carrying Capacity and the Death of a Culture: A Tale of Two
Autopsies. Sociological Inquiry
63:202-223.
-----.
1994. Foundations of Human Ecology. Sociological
Perspectives 37:75-95.
Catton,
W.R., and R.E. Dunlap. 1980. A New Ecological Paradigm for Post-Exuberant
Sociology. American Behavioral
Scientist 24:15-47.
Clark,
P.J., P.T. Eckstrom, and L.D. Linden. 1964. On the Number of Individuals
Per Occupation in a Human Society. Ecology
45:367-372.
Cody,
M.L., and J.M. Diamond, eds. 1975. Ecology
and Evolution of Communities. Cambridge: Belknap Press.
Cohen,
Mark N. 1977. The Food Crisis in
Prehistory: Overpopulation and the Origins of Agriculture. New Haven:
Yale University Press.
Coontz,
S.H. 1961. Population Theories and
the Economic Interpretation. London: Routledge and Kegan Paul.
Crombie,
A.C. 1947. Interspecific Competition. Journal
of Animal Ecology 16:44-73.
Culbertson,
J. 1971. Economic Development: An
Ecological Approach. New York: Alfred A. Knopf.
Dames
& Moore, Inc. 1973. Environmental
Report, Cholla Power Project, Joseph City, Arizona. Phoenix: Arizona
Public Service Company.
Day,
R.H, and T. Grove, eds. 1975. Adaptive
Economic Models. New York: Academic Press.
Eiseley,
L.C. 1958. Darwin's Century. New
York: Doubleday.
Ehrlich,
P.R, and LC. Birch. 1967. The "Balance of Nature" and
"Population Control". The
American Naturalist 101:97-107.
Faris,
J.C. 1975. Social Evolution, Population, and Production. In Population,
Ecology and Social Evolution, edited by S. Polgar, pp. 235-271. The
Hague: Mouton.
Flannery,
K.V. 1972. The Cultural Evolution of Civilizations. Annual
Review of Ecology and Systematics 1:399‑426.
Forrest,
E.R. 1952. Arizona's Dark and Bloody
Ground, Revised Edition. Caldwell, Idaho: Caxton Printers.
Foster,
G.M. 1967. Tzintzuntzan: Mexican
Peasants in a Changing World. Boston: Little, Brown.
Frank,
A.G. 1967. Capitalism and
Underdevelopment in Latin America: Historical Studies of Chile and Brazil.
New York: Monthly Review Press.
Frazer,
J.G. 1890. The Golden Bough. London:
Macmillan.
Fried,
M. 1967. Evolution of Political
Society: An Essay in Political Anthropology. New York: Random House.
Gall,
P.L., and A. Saxe. 1977. The Ecological Evolution of Culture: The State as
Predator in Prehistory. In Exchange
Systems in Prehistory, edited by T. Earle and J.E. Ericson, pp.
255-268. New York: Academic Press.
Gause,
G.F. 1934. The Struggle for
Existence. Baltimore: Williams and Wilkins.
Gibson,
L.J., and R.W. Reeves. 1970. Functional Bases of Small Towns: A Study of
Arizona Settlements. Arizona Review
19:19-26.
Graves,
T.D., N.B. Graves, and M.J. Kobrin. 1969. Historical Inferences from
Guttman Scales: The Return of Age Area Magic? Current
Anthropology 10:317-338.
Greider,
K. 1973. Invitation to Physics. New
York: Harcourt Brace Jovanovich.
Haggett,
P. 1966. Locational Analysis in
Human Geography. London: Halstead.
Hardesty,
D.L. 1977. Ecological Anthropology.
New York: John Wiley and Sons.
Hardin,
G. 1960. The Competitive Exclusion Principle. Science
131:1292-1297.
Harris,
M. 1959. The Economy Has No Surplus? American
Anthropologist 61:185-199
-----.
1964. Patterns of Race in the
Americas. New York: Walker.
-----.
1968. The Rise of Anthropological
Theory. New York: Thomas Y. Crowell.
-----.
1980. Culture, People, Nature, Third
Edition. New York: Harper and Row.
Hempel,
C. 1965. Aspects of Scientific
Explanation: And Other Essays in the Philosophy of Science. New York:
Free Press.
Hogbin,
H.I. 1964. A Guadalcanal Society:
The Kaoka Speakers. New York: Holt, Rinehart and Winston.
Holling,
C.S. 1973. Resilience and Stability of Ecological Systems. Annual Review of Ecology and Systematics 4:1-23.
Hornocker,
M. 1970. An Analysis of Mountain
Lion Predation Upon Mule Deer and Elk in the Idaho Primitive Area.
Wildlife Monograph 22, The Wildlife Society.
Kaplan,
D. 1960. The Law of Cultural Dominance. in M. Sahlins and E. Service, eds.
Evolution and Culture.Ann Arbor:
University of Michigan Press.
Kottak,
C.P. 1982. The Past in the Present:
History, Ecology, and Cultural Variation in Highland Madagascar. Ann
Arbor: University of Michigan Press.
Kummer,
H. 1971. Primate Societies: Group
Techniques of Ecological Adaptation. Chicago: Aldine.
Kunkel,
J. 1970. Society and Economic
Growth: A Behavioral Perspective of Social Change. London: Oxford
University Press.
Lees,
S., and D. Bates. 1984. Environmental Events and the Ecology of
Cummulative Change. In The Ecosystem
Concept in Anthropology, edited by E. Moran, pp. 133-159. Boulder:
Westview Press.
Leigh,
E.G., Jr. 1965. On the Relation between Productivity, Biomass, Diversity
and Stability of a Community. Proceedings
of the National Academy of Sciences 53:777-783.
-----.
1975. Population Fluctuations, Community Stability, and Environmental
Variability. In Ecology and
Evolution of Communities, edited by M.L. Cody and J.M. Diamond, pp.
51-73. Cambridge: Belknap Press.
Leone,
M. 1979. The Roots of Modern
Mormonism. Cambridge: Harvard University Press.
Levins,
R. 1968. Evolution in Changing
Environments. Princeton: Princeton University.
Press.
Lewin,
K. 1935. The Conflict between Aristotelian and Galileian Modes of Thought
in Contemporary Psychology. in K. Lewin, A
Dynamic Theory of Personality. New York: McGraw-Hill, pp. 1-42.
Little,
M.A., and G.E.B. Morren, Jr. 1976. Ecology,
Energetics, and Human Variability. Dubuque, Iowa: Wm. C. Brown.
MacArthur,
R.H. 1972. Geographical Ecology.
New York: Harper and Row.
MacArthur,
R., and J. MacArthur. 1961. On Bird Species Diversity. Ecology
42:594-598.
Margalef,
R. 1968. Perspectives in Ecological
Theory. Chicago: University of Chicago Press.
May,
R.M. 1973. Stability and Complexity
in Model Ecosystems. Princeton: Princeton University Press.
Maine,
H.S. 1861. Ancient Law. London:
J. Murray.
Meggers,
B.J. 1971. Amazonia: Man and Culture
in a Counterfeit Pardise. Chicago: Aldine‑Atherton.
Moran,
E. ed. 1984. The Ecosystem Concept
in Anthropology. Boulder: Westview Press.
Morgan,
L.H. 1877. Ancient Society. New
York: Holt, Rinehart and Winston.
Moynihan,
D.P. 1965. The Negro Family: The
Case for National Action. Washington, D.C.: Office of Policy Planning
and Research, U.S. Department of Labor.
Nag,
M., B. White, and R.C. Post. 1978. An Anthropological Approach to the
Study of the Economic Value of Children in Java and Nepal. Current
Anthropology 19:293-306.
Nagel,
E. 1979. The Structure of Science:
Problems in the Logic of Scientific Explanation. Hackett:
Indianapolis.
Netting,
R.M. 1968. Hill Farmers of Nigeria:
Cultural Ecology of the Kofyar of the Jos Plateau. Seattle: University
of Washington Press.
Nisbet,
R.A. 1969. Social Change and
History: Aspects of the Western Theory of Development. London: Oxford
University Press.
Odum,
E.P. 1971. Fundamentals of Ecology,
Third Edition. Philadelphia: Saunders.
Odum,
H.T. 1971. Environment, Power and
Society. New York: John Wiley and Sons.
Odum,
H.T., and R.C. Pinkerton. 1955. Times Speed Regulator: the Optimum
Efficiency for Maximum Output in Physical and Biological Systems. American
Scientist 43:331-343.
Paine,
R.T. 1966. Food Web Complexity and Species Diversity. The American
Naturalist 100:65-75.
Peterson,
C.S. 1973. Take Up Your Mission:
Mormon Colonizing along the Little Colorado River 1870‑1900.
Tucson: University of Arizona Press.
Pianka,
E.R. 1966. Latitutinal Gradients in Species Diversity: A Review of
Concepts. The American Naturalist
100:33-46.
Piddocke,
S. 1965. The Potlatch System of the Southern Kwakiutl. Southwestern Journal of Anthropology 21:244-264.
Pielou,
E.C. 1975. Ecological Diversity.
New York: Wiley.
Plog,
F.T. 1973. Diachronic Anthropology. In Research
and Theory in Current Archaeology, edited by C. Redman. New York: John
Wiley and Sons.
Rappaport,
R. 1968. Pigs for the Ancestors:
Ritual in the Ecology of a New Guinea People. New Haven: Yale
University Press.
Rapport,
D.J., and J.E. Turner. 1977. Economic Models in Ecology. Science 195:367-373.
Ricklefs,
R.E. 1987. Community Diversity: Relative Roles of Local and Regional
Processes. Science 235:167‑171.
Rodhe,
W. 1955. Can Plankton Production Proceed during Winter Darkness in
Subarctic Lakes? Proceedings of the
International Association of Theoretical and Applied Limnologists
12:117-122.
Rose,
D. 1981. Energy Transition and the
Local Community: A Theory of Society Applied to Hazleton, Pennsylvania.
Philadelphia: University of Pennsylvania Press.
Rosenzweig,
M.L. 1968. Net Primary Productivity of Terrestrial Communities:
Predictions from Climatological Data. American
Naturalist 102:67-74.
-----.
1976. On Continental Steady States of Species Diversity. In Ecology
and Evolution of Communities, edited by M.L. Cody and M. Diamond, pp.
121-140. Cambridge: Belknap Press.
Russo,
J. 1964. The Kaibab North Deer Herd:
Its History, Problems and Management. State of Arizona Game and Fish
Department, Bulletin 7.
Sahlins,
M.D. 1958. Social Stratification in
Polynesia. Seattle: University of Washington Press.
-----.
1961. The Segmentary Lineage: An Organization of Predatory Expansion. American
Anthropologist 63:322-343.
Sahlins,
M.D., and E. Service. 1960. Evolution
and Culture. Ann Arbor: University of Michigan Press.
Samuelson,
P. 1958. Economics:
An Introductory Analysis, Fourth Edition. New York: McGraw‑Hill.
Sanders,
H.L. 1968. Marine Benthic Diversity: A Comparative Study. The
American Naturalist 102:243-282.
Sanders,
W.T., and D.L. Nichols. 1988. Ecological Theory and Cultural Evolution in
the Valley of Oaxaca. Current
Anthropology 29:33-80.
Sanders,
W.T., and B.J. Price. 1968. Mesoamerica:
The Evolution of A Civilization. New York: Random House.
Schoener,
T.W. 1971. Large-Billed Insectivorous Birds: A Precipitous Diversity
Gradient. Condor 73:154-161.
Service,
E. 1971. Primitive Social
Organization: An Evolutionary Perspective, Second Edition. New York:
Random House.
Shannon,
F.A. 1945. The Farmer's Last
Frontier, Agriculture, 1960-1897.
New York: Holt, Rinehart and Winston.
Simon,
J.L. 1977. The Economics of
Population Growth. Princeton: Princeton University Press.
Slobodkin,
L.B., and H.L. Sanders. 1969. On the Contribution of Environmental
Predictability to Species Diversity. Diversity
and Stability in Ecological Systems. Brookhaven Symposia in Biology
22:82-95.
Smith,
C.A. ed. 1976. Regional Analysis,
Volumes I and II. New York: Academic Press.
Smith,
E.A. 1984. Anthropology, Evolutionary Ecology, and the Explanatory
Limitations of the Ecosystem Concept. In The
Ecosystem Concept in Anthropology, edited by E. Moran, pp. 51-86.
Boulder: Westview Press.
Spencer,
H. 1876. Principles of Sociology. New
York: D. Appleton.
Stocking,
G.W. Race, Culture and Evolution:
Essays in the History of Anthropology. New York: Free Press.
Steward,
J. 1955. Theory of Culture Change:
The Methodology of Multilinear Evolution. Urbana: University of
Illinois Press.
Terborgh,
J. 1971. Distribution on Environmental Gradients: Theory and a Preliminary
Interpretation of Distributional Patterns in the Avifauna of the
Cordillera Vilcabama, Peru. Ecology
52:23-40.
Thomas,
E.N. 1960. Some Comments on the Functional Bases for Small Iowa Towns. Iowa
Business Digest 31:10-16.
Tylor,
E.B. 1871. Primitive Culture.
London: John Murray.
Vandermeer,
J.H. 1972. Niche Theory. Annual
Review of Ecology and Systematics 3:107-132.
Vayda,
A., and B.J. McCay. 1975. New Directions in Ecology and Ecological
Anthropology. Annual Review of
Anthropology 4:297-306.
Vayda,
A., and R. Rappaport. 1968 Ecology:
Cultural and Non‑Cultural. In Introduction
to Cultural Anthropology, edited by J.A. Clifton, pp. 477-497. New
York: Houghton‑Mifflin.
VonBertalanffy,
L. 1968. General Systems Theory.
New York: Braziller.
White,
B. 1973. Demand for Labor and Population Growth in Colonial Java. Human Ecology 1:217-236.
-----.
1975. The Economic Importance of Children in a Javanese Village. In Population and Social Organization, edited by M. Nag, pp. 127-146.
The Hague: Mouton.
White,
L.A. 1949. The Science of Culture.
New York: Grove Press.
-----.
1959. The Evolution of Culture.
New York: McGraw-Hill.
Whittaker,
R.H. 1975. Communities and
Ecosystems, Second Edition. New York: Macmillan.
Whittaker,
R.H., and G.M. Woodwell. 1972. Evolution of Natural Communities. In Ecosystem Structure and Function, edited by J.A. Weins, pp. 137-159.
Eugene: University of Oregon Press.
Wilkinson,
R. 1973. Poverty and Progress: An
Ecological Perspective on Economic Development. New York: Praeger.
Wilson,
F. 1969. Explanation in Aristotle, Newton and Toulmin. Philosophy
of Science 36:291-310.
Wilson,
E.O. 1968. The Ergonomics of Caste in the Social Insects. American
Naturalist 102:41-66.
-----.
1971. The Insect Societies.
Cambridge: Belknap Press.
Winterhalder,
B., and E. Smith, eds. 1981. Hunter-Gatherer
Foraging Strategies: Ethnographic and Archaeological Analyses.
Chicago: University of Chicago Press.
Woodruff
Irrigation and Recreation Project. n.d. Woodruff Irrigation and Recreation
Program. Woodruff, Arizona.
Young, G., and C.A. Broussard. 1986. The Species Problem in Human Ecology. In Human Ecology: A Gathering of Perspectives, edited by R.J. Borden, pp.55-67. College Park, Maryland: Society for Human Ecology.
.jpg)
